Background
Measurement of liver iron concentration (LIC) by MRI is recommended in several guidelines on the management of iron overload in patients with thalassemia. However, a key barrier to adoption of such methods in resource poor countries is access to expertise and training in the associated image data analysis [Padeniya, et al. (2020) Orphanet Journal of Rare Diseases, 15: 26]. A new software system, DLA R2-MRI, based on deep-learning trained neural networks, (FerriSmart®, Resonance Health Analysis Services Pty Ltd, Australia) has become available that automatically carries out both input data quality assurance and data analysis of suitable MR images, increasing the possibility of more widespread adoption of reliable MRI based LIC measurement.
Aims
The aim of this study was to assess the diagnostic accuracy of the index DLA R2-MRI method on MR images acquired as part of a previous study comparing phlebotomy with deferasirox for the treatment of iron overload in pediatric patients with thalassemia major following curative stem cell transplantation [Inati, et al. (2017) Pediatric Blood & Cancer, 64: 188]. LIC values from MR scans obtained with the spin-density-projection-assisted R2 MRI method (FerriScan®) were to be used as the reference values for the diagnostic accuracy study.
Methods
Archived MR data from the previous study were retrieved and analysed using the fully automated DLA R2-MRI software to generate LIC values. No human input was required in the analysis process and results were generated in seconds. The methods of Bland and Altman were used to determine the bias and 95% limits of agreement between the index and reference method. None of the image datasets in the study had been used to train the DLA R2-MRI neural networks. The sensitivities and specificities (with 95% CIs) of the index method for predicting LIC values by the reference method above the clinically relevant thresholds of 3, 5, 7, and 15 mg Fe/g dw were calculated using the Wilson-Brown method.
Results
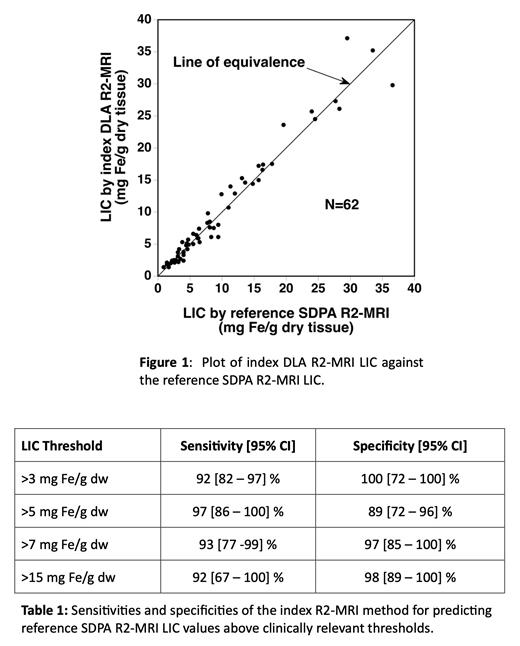
Sixty nine (69) evaluations of LIC were carried out in the original study using the reference method. Sixty eight (68) datasets were retrieved from archives with one dataset missing. The 68 datasets were analysed by the DLA R2-MRI software. Six were rejected by the input quality control module owing to an absent date of birth in the DICOM images. The remaining 62 datasets yielded an LIC value. The 62 successful analyses represented evaluations on 32 patients with 8 patients being scanned 3 times, 14 patients being scanned twice, and 10 patients being scanned once. Patients (18 male, 14 female) ranged in age from 3.2 to 19.7 years at first scan (mean 13.1 SD 4.1 years). Twenty six (26) patients underwent iron reduction therapy during the study (14 phlebotomy and 12 chelation); 5 patients did not receive iron reduction therapy (4 not meeting eligibility criteria, and one was not treated due to parental refusal); one patient withdrew from the study after only 2 months of iron reduction therapy. A plot of the 62 LIC values by the index method plotted against the values from the reference method is shown in Figure 1. The bias between the index and reference results represented as the geometric mean ratio of the index LIC to the reference LIC was statistically insignificant being 1.00 [95% CI 0.96 - 1.05]. The sensitivities and specificities of the index method for predicting reference LIC values above clinically relevant thresholds are shown in Table 1.
Conclusions
The data show a negligible bias between the fully automated index DLA R2-MRI method and the manual expert-dependent reference SDPA R2-MRI method. The diagnostic accuracy is sufficient to guide iron reduction therapy in thalassemia major patients. The ability of the software both to provide input data quality assessment and LIC results within seconds without the need for data analyst training or outsourced analysis offers the opportunity for lower cost and more widespread availability of reliable LIC measurement by MRI for thalassemia major patients in Lebanon and beyond.
Disclosures
Taher:Pharmacosmos: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Vifor: Consultancy, Research Funding; Bristol Myers Squibb (Celgene): Consultancy, Research Funding; Novartis Pharmaceuticals: Consultancy, Research Funding. St Pierre:Resonance Health Analysis Services Pty Ltd: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal